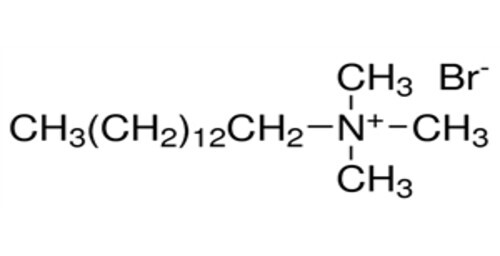Call : 08045801870
Cetrimide EP
660 INR/Kilograms
Product Details:
- Storage Other
- Type Other
- Grade Other
- Physical Form Other
- Click to view more
X
Cetrimide EP Price And Quantity
- 400.00 - 500.00 INR/Kilograms
- 100 Kilograms
- 660 INR/Kilograms
Cetrimide EP Product Specifications
- Other
- Other
- Other
- Other
Cetrimide EP Trade Information
- 100000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
As the topmost manufacturer, exporter and ïsupplier, our organization is engaged in offering Cetrimide EP. The provided solution is suitable for preventing infections from bacteria and fungal by making them inactive. This solution processed in conformance with market standards by utilizing supreme quality chemicals and ultramodern processing techniques. Available in various packaging options, this Cetrimide EP can be purchased from us at market leading prices.
Features:
- Free from toxic elements
- Enhanced shelf life
- High effectiveness
- Precise composition
- Cetrimide EP refers to Cetrimide European Pharmacopoeia The European Pharmacopoeia EP sets the standards and specifications for medicines and pharmaceutical substances in European countries Therefore Cetrimide EP indicates that cetrimide complies with the quality standards established by the European Pharmacopoeia for pharmaceutical use
-
- Similar to Cetrimide IP Indian Pharmacopoeia Cetrimide EP is primarily used as an antiseptic agent in various pharmaceutical and healthcare products It exhibits broadspectrum antimicrobial activity against bacteria fungi and some viruses Here are some common uses of Cetrimide EP
-
- 1 Antiseptic Solutions It is a key ingredient in antiseptic solutions used for disinfecting and cleaning wounds cuts and burns
-
- 2 Topical Creams and Ointments Cetrimide EP can be found in medicated creams and ointments used for treating skin infections and promoting wound healing
-
- 3 Shampoos It is used in medicated shampoos to treat scalp conditions like dandruff
-
- 4 Ophthalmic Preparations In eye drops and solutions Cetrimide EP helps prevent and treat eye infections
-
- Cetrimide EP works by disrupting the cell membranes of microorganisms which leads to their death or inhibition of growth It is generally welltolerated when used as directed but like any medication it can cause irritation or allergic reactions in some individuals The European Pharmacopoeia ensures that Cetrimide EP meets stringent quality standards regarding purity safety and effectiveness for pharmaceutical applications in Europe
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Cetrimide Powder' category
 |
BASIC PHARMA LIFE SCIENCE PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |

 Send Inquiry
Send Inquiry